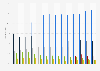
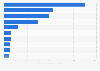
Share of clinical trials in selected countries APAC 1999-2024, by health category
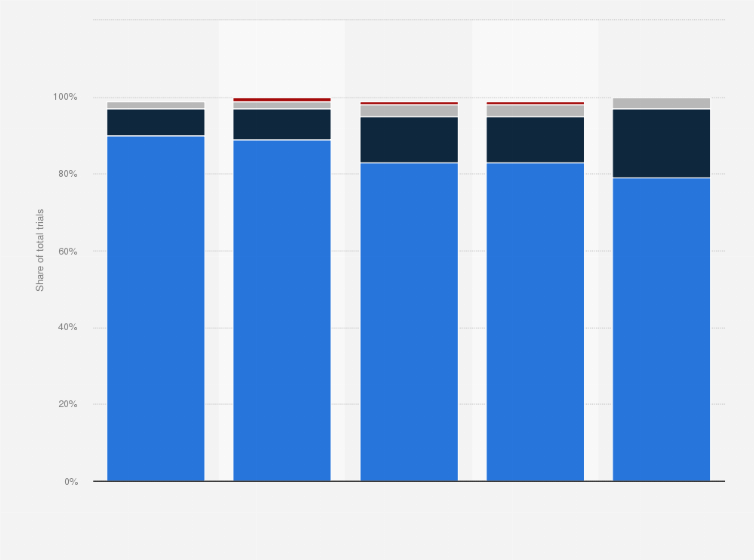
Between 1999 and 2024 (as of June 2024), around 83 percent of clinical trials in China were for pharma products for non-communicable diseases. Around 18 percent of clinical trials in India were for communicable, maternal, perinatal, and nutritional conditions.
What are non-communicable diseases?
Non-communicable diseases (NCDs), often referred to as chronic diseases, are noninfectious health conditions that are typically long-lasting and progress overtime. NCDs are often influenced by lifestyle, environmental factors, genetics, and aging. The main types of NCDs include cardiovascular diseases (such as heart attacks and stroke), cancers, chronic respiratory diseases (such as chronic obstructive pulmonary disease and asthma) and diabetes. Among these NCDs, cancer is the leading focus area of pharmaceutical research and development worldwide.
Scale of clinical trials in China
China is a powerhouse in clinical research, conducting over 135,000 trials between 1999 and 2024, far surpassing other countries in the Asia-Pacific region. The majority of clinical trials in China are relatively small in scale, with nearly half of the trials involving fewer than 100 participants. Yet, around 76 clinical trials with more than 500,000 participants were conducted in China during this period, more than double the amount of clinical trials of this scale in Japan.